The in-vitro medical devices Regulation (IVDR) is the new harmonized regulatory framework to ensure the safety and performance of in-vitro diagnostic medical devices on the European market. The IVDR will replace the EU's current Directive on in vitro diagnostic medical devices (IVDD 98/79/EC)
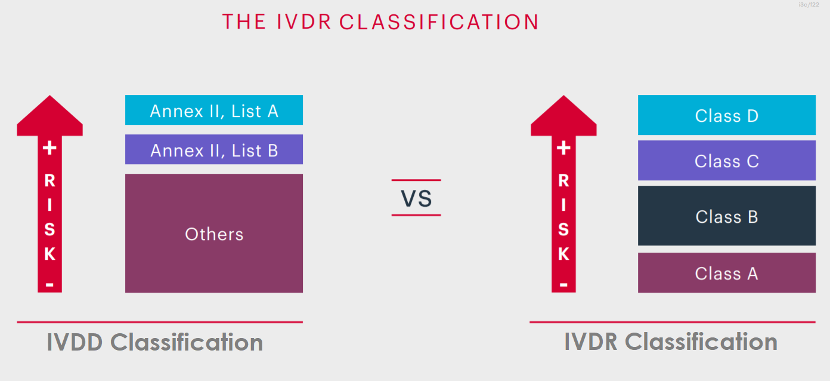
IVDR Classification is based on the intended purpose and inherent risks of In-Vitro Diagnostic Devices (IVDs), therefore they are classified into classes A, B, C and D considering their intended purpose and inherent risks.